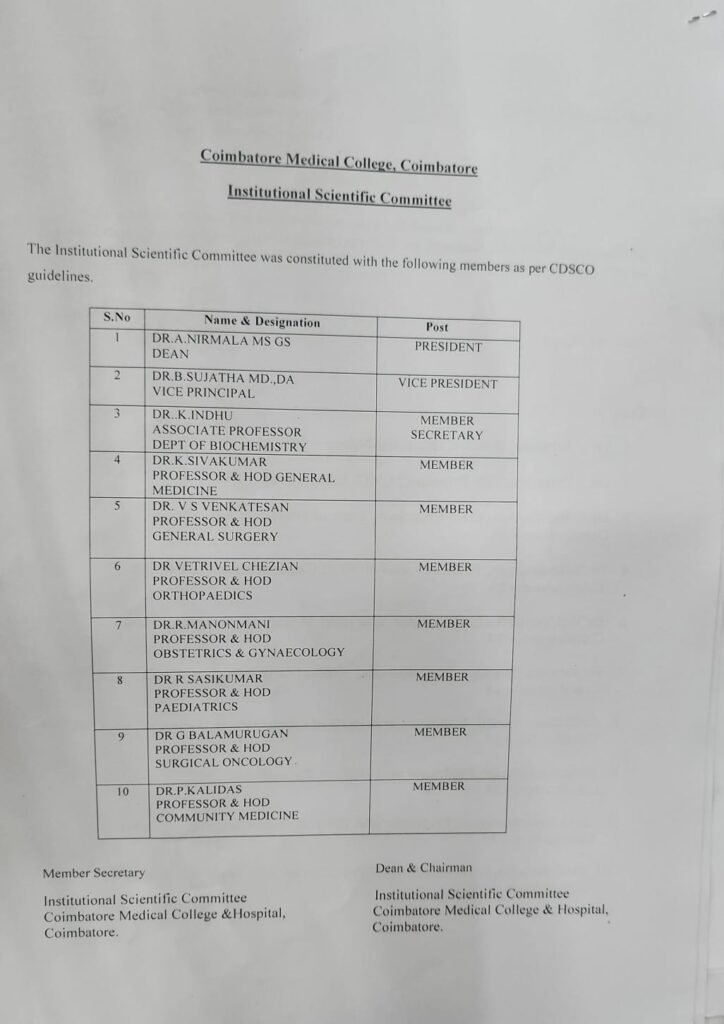
🛡️ Institution Human Ethics Committee (IHEC) – Roles & Responsibilities
An Institutional Human Ethics Committee (IHEC) is responsible for reviewing, approving, and monitoring biomedical and health research involving human participants. Its primary goal is to ensure that the research is conducted ethically, with respect for the dignity, rights, safety, and well-being of participants.
1. Scientific and Ethical Review of Research Proposals
- Evaluate the scientific validity of research to ensure it is methodologically sound.
- Assess ethical issues, including risk-benefit analysis, voluntariness, and participant safety.
- Ensure informed consent processes are clear, comprehensive, and culturally appropriate.
2. Safeguarding Participant Rights and Welfare
- Protect the privacy and confidentiality of participants.
- Ensure vulnerable groups (e.g., children, pregnant women, mentally impaired individuals) are given special protection.
- Promote justice and equity in participant selection and treatment.
3. Approval and Oversight
- Provide initial approval for research before it begins.
- Conduct continuing review of ongoing research to monitor compliance.
- Evaluate protocol deviations or changes and decide on their acceptability.
4. Documentation and Record-Keeping
- Maintain records of all submitted documents, meeting minutes, decisions, and correspondence.
- Ensure that documentation is accurate, confidential, and retained for the required period.
5. Ethics Education and Guidance
- Promote awareness of ethical principles among researchers.
- Provide guidance on Good Clinical Practice (GCP) and national/international ethical guidelines (e.g., ICMR, CIOMS, Declaration of Helsinki).
6. Addressing Complaints and Violations
- Investigate and respond to any ethical concerns or complaints raised by participants or third parties.
- Recommend actions in case of non-compliance or misconduct, including suspension or termination of studies.